Welcome to the third edition of the Athena BDA Newsletter. Each month we bring you a summary of all the high priority FDA approvals, new indication approvals and flag pipeline blockbuster assets that have submission updates or positive phase 2/3 data announcements.
Each month we spend days trawling through the deepest recesses of the internet to build a news service identifying high priority targets for folks selling into pharma. Additionally, we prioritize each asset based on expected peak sales and how many contacts are identifiable to approach.
No more Global Data daily updates about a phase 1 asset in Birdshot Chorioretinopathy or launch news from Drugs.com* about some lame generic from Dr Reddy’s. Just subscribe to us. Let us graft through countless news sources to identify the hottest assets then you take the glory 18 months from now when contracting finally closes.
Why are we doing this you ask? Well, we also hand deliver our clients over 500 contacts “potentially working” and 150 “exact match” contacts who definitely work on these high priority assets. If that sounds of interest, please get in touch.
Now on to the updates. This month we identified 7 new FDA approvals, 4 new indication approvals and 20 new pipeline blockbuster assets. See summaries below for all priority 1 and 2 assets with a table containing the rest of the priority 3 assets.
Three companies/divisions we’re very hot on are Avidity, PTC, and AbbVie Neuroscience. Read below to see why.
Pipeline Blockbuster Updates:
Survodutide – Boehringer Ingelheim – MASH
The FDA has granted Breakthrough Therapy designation to Boehringer Ingelheim’s survodutide for treating MASH with moderate to advanced fibrosis, based on promising Phase II results. Boehringer has launched two Phase III trials, LIVERAGE and LIVERAGE-Cirrhosis, to further investigate survodutide’s impact on disease progression, aiming to address a high unmet need in this progressive liver disease.
Athena BDA priority score of 1 given the multiple billion dollar projected sales, identifiable survodutide contacts and wider range of metabolic leads to approach.
Acoramidis – BridgeBio – Transthyretin Amyloid Cardiomyopathy (ATTR-CM)
BridgeBio’s post-hoc analysis of the Phase 3 ATTRibute-CM trial indicates that acoramidis significantly reduced all-cause mortality and recurrent cardiovascular hospitalizations in ATTR-CM patients. These findings bolster the company’s New Drug Application (NDA), potentially introducing a new treatment option for this life-threatening condition.
Athena BDA priority score of 1 given expected sales of close to $3B, large number of target contacts, and open marketing/ medical job postings.
Teliso-V – AbbVie – Non-Small Cell Lung Cancer (NSCLC)
AbbVie has filed a Biologics License Application (BLA) with the FDA for Teliso-V to treat c-Met overexpressing, EGFR wild-type, non-squamous NSCLC. Supported by positive Phase II results with a 35% response rate in c-Met high patients, this antibody-drug conjugate may offer a new targeted option for previously treated advanced NSCLC, with an ongoing Phase III confirmatory trial.
Athena BDA priority score of 1 given the target patient population and high number of Oncology focussed contacts to approach. Low number of contacts confirmed working on the asset.
Del-desiran – Avidity Biosciences – Myotonic Dystrophy Type 1 (DM1)
The FDA has lifted the partial clinical hold on Avidity Biosciences’ investigational therapy del-desiran, enabling resumption of the Phase III HARBOR trial for DM1. This follows safety adjustments after a serious adverse event reported in an earlier trial, allowing Avidity to continue advancing this potential therapy for the rare muscular disorder.
Athena BDA Priority score of 1 given $1B peak expected sales and large commercial team established and being built.
Athena BDA Hot Tip Alert: There is a HUGE hiring surge at Avidity for numerous patient support, medical and marketing roles, especially within DMD for another phase 3 asset they have called del-zota.
Translarna – PTC Therapeutics – DMD
PTC Therapeutics announced that the FDA has accepted the resubmission of the New Drug Application (NDA) for Translarna (ataluren) for the treatment of DMD. This milestone brings the therapy closer to U.S. approval, potentially offering a new treatment option for patients with this specific genetic mutation.
Athena BDA priority score of 1 given impressive Ex-US sales of over $300M, overall strength of the PTC pipeline, and large influx of commercial contacts into the business in the last 3 months. DMD commercial leads identified.
Sepiapterin – PTC Therapeutics – Phenylketonuria (PKU)
PTC Therapeutics announced that the FDA has accepted the New Drug Application (NDA) for sepiapterin, an oral therapy for pediatric and adult patients with PKU. This acceptance marks a significant step toward providing a new treatment option for PKU patients, potentially addressing unmet medical needs in this community.
Athena BDA priority score of 2 given the overall strength of the PTC pipeline, and large influx of commercial contacts into the business in the last 3 months, but modest expected sales of $150M.
Vatiquinone – PTC Therapeutics – Friedreich Ataxia (FA)
PTC Therapeutics announced positive results from long-term studies of vatiquinone in Friedreich ataxia patients, showing a statistically significant slowing of disease progression over 144 weeks. Following alignment with the FDA, PTC plans to submit a New Drug Application (NDA) by December 2024, potentially offering a new treatment option for this debilitating condition.
Athena BDA priority score of 2 given the overall strength of the PTC pipeline and large influx of commercial contacts into the business in the last 3 months.
UGN-102 – UroGen – Bladder Cancer
The FDA has accepted UroGen’s NDA for UGN-102, a mitomycin formulation using RTGel technology, aimed at treating low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC). Backed by positive Phase III ENVISION trial results, UGN-102 offers a non-surgical treatment alternative by prolonging drug exposure in the bladder, with potential to address a significant unmet need in this patient population.
Athena BDA priority score of 1 given potential market size and established commercial team around the asset.
Clesrovimab – Merck – RSV Vaccine
Merck’s investigational monoclonal antibody, clesrovimab, demonstrated a 60.4% reduction in RSV-associated infections and an 84.2% decrease in related hospitalizations in infants during a Phase 2b/3 trial. These results position clesrovimab as a potential competitor to existing RSV preventive therapies, with Merck planning regulatory submissions in 2025.
Athena BDA priority score of 1 given ~$1B sales of Beyfortus (another RSV vaccine), large commercial team confirmed working on the asset, and a large number of Vaccine targets to approach at Merck.
Delgocitinib – LEO Pharma – Chronic Hand Eczema
LEO Pharma’s New Drug Application (NDA) for delgocitinib cream 2% has been accepted by the FDA for the treatment of moderate to severe chronic hand eczema. This topical pan-Janus kinase inhibitor has demonstrated efficacy and safety in Phase 3 trials, offering a potential new treatment option for patients with this challenging condition.
Athena BDA priority score of 1 given potential market size and identified commercial leadership to approach.
TNX-102 SL – Tonix Pharmaceuticals – Fibromyalgia
Tonix Pharmaceuticals has submitted an NDA to the FDA for TNX-102 SL, a non-opioid, centrally-acting analgesic designed to reduce widespread pain in fibromyalgia. Supported by two successful Phase 3 trials and granted Fast Track designation, TNX-102 SL could become the first new fibromyalgia treatment in over 15 years if approved.
Athena BDA priority score of 2 given unknown market size but senior commercial team is established.
PZ-Cel – Abeona Therapeutics – Epidermolysis Bullosa
Abeona Therapeutics has resubmitted its BLA to the FDA for prademagene zamikeracel (PZ-Cel), a gene therapy for RDEB, addressing FDA-requested CMC items. With support from the Phase 3 VIITAL study, Abeona awaits a new PDUFA date, aiming to offer a first-of-its-kind gene therapy for this rare and painful skin disorder.
Athena BDA priority score of 2 given high unmet need and moderate number of launch contacts to approach.
NTLA-2002 – Intellia – Hereditary Angioedema (HAE)
Intellia Therapeutics’ in vivo CRISPR therapy, NTLA-2002, demonstrated an 81% reduction in monthly HAE attacks in a Phase 2 trial, with 8 of 11 patients on the 50 mg dose remaining attack-free over a median follow-up of eight months. These results support plans to initiate a Phase 3 trial by the end of 2024, aiming to offer a single-dose, long-term treatment option for HAE patients.
Athena BDA priority score of 2 given potential market opportunity and moderate number of launch contacts to approach. Also have another phase 3 asset called NTLA-2001 which could gain approval.
Bitopertin – Disc Medicine – Erythropoietic Protoporphyria (EPP)
Disc Medicine is preparing to file an NDA with the FDA for bitopertin to treat EPP, a rare genetic disorder causing severe photosensitivity. Supported by promising data showing reduced pain and skin damage in EPP patients, bitopertin could become the first approved treatment specifically for this condition.
Athena BDA priority score of 2 given potential peak sales of $600M and moderate number of launch contacts to approach. Currently hiring for a VP of Marketing and multiple Medical Leads.
FDA Approval Updates:
HYMPAVZI – Pfizer – Hemophilia A and B
Pfizer has received FDA approval for HYMPAVZI, a once-weekly subcutaneous treatment for hemophilia A and B patients without inhibitors, marking its second hemophilia therapy approval this year. Administered via a pre-filled auto-injector pen, HYMPAVZI demonstrated a 92% reduction in bleeding episodes over 12 months compared to standard treatments, offering a convenient alternative to frequent intravenous infusions.
Athena BDA priority score of 1 given the large number of launch contacts to approach and strong hemophilia pipeline including Beqvev, which launched earlier this year. Relatively modest expected peak sales of $300M.
Vyalev – AbbVie – Parkinson’s Disease
AbbVie has received FDA approval for Vyalev, a 24-hour subcutaneous infusion therapy combining foscarbidopa and foslevodopa, designed to manage motor fluctuations in advanced Parkinson’s disease. This approval follows two prior rejections and offers a more convenient alternative to existing treatments, potentially enhancing patient adherence and quality of life.
Athena BDA priority score of 1 given expected peak sales of close to $1B and large number of contacts confirmed working on the asset and wider group of Parkinson’s and Neuroscience contacts to approach.
Athena BDA Hot Tip Alert: AbbVie’s Neuroscience pipeline is stacked having just launched Vyalev, and with two additional pipeline blockbusters, tavapadon and emraclidine, from the recent acquisition of Cerevel.
Vyloy – Astellas – Gastric Cancer
Astellas has secured FDA approval for Vyloy, a first-in-class therapy targeting CLDN18.2-positive, HER2-negative gastric cancer, to be used in combination with chemotherapy. This approval follows earlier endorsements in Japan and the UK, positioning Vyloy as a significant advancement in treating this challenging cancer subtype.
Athena BDA priority score of 1 given expected peak sales of $800M and large number of contacts confirmed working on the asset and wider group of Oncology contacts to approach.
Itovebi – Roche – HR+/HER2- Breast Cancer
Roche has received FDA approval for Itovebi, a PI3K inhibitor, for use in combination with Ibrance and Faslodex in HR+/HER2- advanced or metastatic breast cancer patients with PIK3CA mutations. This approval positions Itovebi as a competitor to Novartis’ Piqray and AstraZeneca’s Truqap, potentially enhancing Roche’s breast cancer portfolio.
Athena BDA priority score of 1 with peak expected sales of close to $2B and a large number of Breast Cancer and Oncology contacts to approach, however no contacts identified as definitely working on the asset.
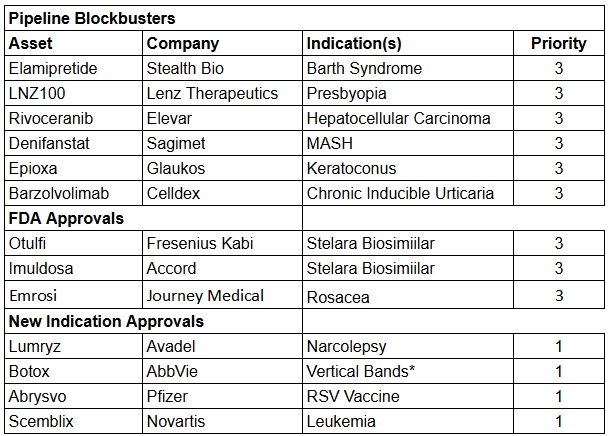
The rest….
*FYI – Drugs.com is an amazing source that I would highly recommend to any pharma BD lead!

