Welcome to the fourth edition of the Athena BDA Newsletter. Each month we bring you a summary of all the high priority FDA approvals, new indication approvals and flag pipeline blockbuster assets that have submission updates or positive phase 2/3 data announcements.
We dedicate countless hours exploring the farthest corners of the internet to deliver a news service that highlights high-priority assets—so you don’t have to.
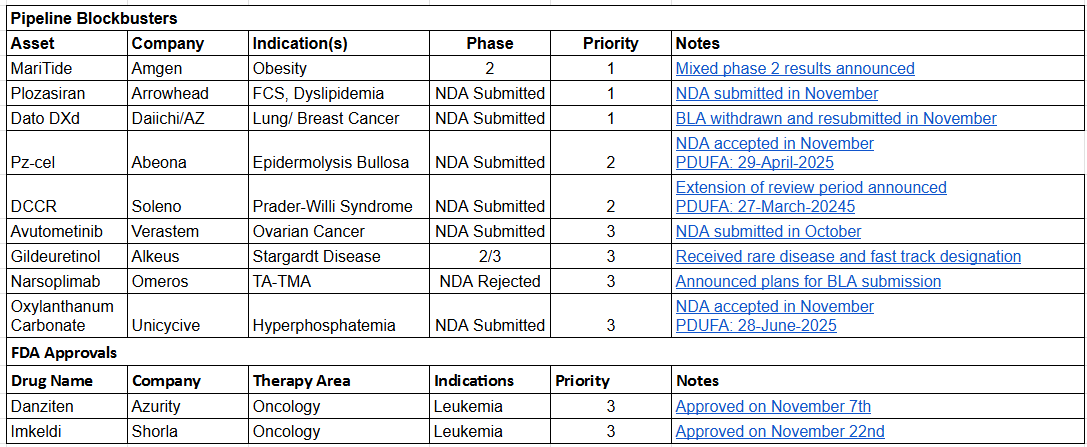
Now on to the updates. This month we’ve highlighted 7 new FDA approvals, 1 new indication approval and 22 pipeline assets, all with November news catalysts such as NDA submissions or positive trial results.
***Three companies/assets we’re very hot on are Axsome, Rilzabrutinib and Bimzelx. Read below to see why.
Pipeline Blockbuster Updates:
Icotrokinra – J&J – Plaque Psoriasis
Johnson & Johnson’s investigational oral peptide, icotrokinra, achieved significant skin clearance in a Phase 3 trial for moderate-to-severe plaque psoriasis, with 64.7% of patients attaining clear or almost clear skin at week 16. This positions icotrokinra as a potential competitor to Bristol Myers Squibb’s Sotyktu, with J&J planning further studies to directly compare their efficacy.
Athena BDA priority score of 1 given peak potential sales of $5B. Only 2 launch contacts identified but many immunology focused contacts to approach.
Sibeprenlimab – Otsuka – IgA Nephropathy
Otsuka’s investigational anti-APRIL monoclonal antibody, sibeprenlimab, achieved a statistically significant and clinically meaningful reduction in 24-hour urine protein-to-creatinine ratio compared to placebo after nine months in a Phase 3 trial for IgA nephropathy. Otsuka plans to discuss these interim results with the FDA to explore potential accelerated approval, with final study outcomes expected in early 2026.
Athena BDA priority score of 1 given peak estimated sales approaching $1B, launch leads identified and a range of nephrology targets to approach given Otsuka’s presence in this space (Jynarque).
Rilzabrutinib – Sanofi – ITP
Sanofi’s oral Bruton tyrosine kinase inhibitor, rilzabrutinib, achieved a durable platelet response in 23% of patients with persistent or chronic immune thrombocytopenia in the Phase 3 LUNA 3 trial, compared to none in the placebo group. The company plans to present detailed results at the American Society of Hematology Annual Meeting later this week.
Athena BDA priority score of 1 given large market potential and high number of launch contacts identified.
Athena BDA Hot Tip Alert:
Given the positive reception to this announcement and the significant number of launch contacts assigned to this asset, we are designating it as one of our highly sought after Hot Tip Alerts😉 .
Blenrep – GSK – Multiple Myeloma
GSK’s Blenrep, previously withdrawn in 2022 after failing to meet confirmatory trial requirements, has shown a significant survival benefit in the Phase III DREAMM-7 trial, outperforming J&J’s Darzalex in relapsed or refractory multiple myeloma. The FDA has accepted GSK’s Biologics License Application for Blenrep combinations, with a decision expected by July 23, 2025.
Athena BDA priority score of 1 given the $4B peak sales estimate and large number of multiple myeloma contacts to approach within GSK.
Nipocalimab – J&J – Sjogren’s Disease, Myasthenia Gravis
The FDA has granted Breakthrough Therapy Designation to J&J’s investigational anti-FcRn antibody, nipocalimab, for treating adults with moderate-to-severe Sjogren’s disease, a chronic autoimmune disorder affecting approximately four million people worldwide. Nipocalimab is also in development for myasthenia gravis.
Athena BDA priority score of 1 given $5B peak potential sales with numerous launch leads identified and a larger number of immunology and neuroscience contacts to approach.
Zanzalintinib – Exelixis – Head and Neck Cancer, Renal Cell Carcinoma
Exelixis and Merck have entered a clinical development collaboration to evaluate Exelixis’ investigational tyrosine kinase inhibitor, zanzalintinib, in combination with Merck’s Keytruda for head and neck squamous cell carcinoma, and with Welireg for renal cell carcinoma. This partnership includes a Phase 3 trial for head and neck cancer and multiple trials for renal cell carcinoma, aiming to enhance treatment options for these cancers.
Athena BDA priority score of 1 given peak expected sales approaching $1B, confirmation Exelixis will maintain marketing rights, and a confirmed launch lead identified.
Donidalorsen – Ionis – Hereditary Angioedema
The FDA has accepted Ionis’ New Drug Application for donidalorsen, an investigational RNA-targeted therapy aimed at preventing attacks of Hereditary Angioedema in patients aged 12 and older. The agency has set a Prescription Drug User Fee Act action date of August 21, 2025.
Athena BDA priority score of 2 given $700M peak potential sales with moderate to low number of commercial contacts to approach but with multiple open job postings for HAE roles.
Lete-cel – Adaptimmune – Sarcoma
Adaptimmune’s investigational T-cell therapy, lete-cel, achieved a 42% overall response rate in the pivotal Phase 2 IGNYTE-ESO trial for advanced synovial sarcoma and myxoid/round cell liposarcoma. Based on these results, the company plans to initiate a rolling Biologics License Application submission by the end of 2025.
Athena BDA priority score of 2 given peak expected sales of the sarcoma franchise to reach $400M and a moderate number of commercial contacts to approach.
AXS-12 – Axsome – Narcolepsy
Axsome’s investigational therapy, AXS-12 , achieved the primary endpoint in the Phase 3 ENCORE trial by significantly reducing the frequency of cataplexy attacks in narcolepsy patients compared to placebo. The company plans to request a pre-NDA meeting with the FDA to expedite the New Drug Application filing for AXS-12.
Athena BDA priority score of 2 given peak potential sales of $500M. No confirmed contacts working on this asset but a large set of contacts to approach.
AXS-07 – Axsome – Migraine
The FDA has accepted Axsome’s resubmitted New Drug Application for AXS-07, an oral treatment for acute migraine, setting a Prescription Drug User Fee Act action goal date of January 31, 2025. This resubmission follows a previous complete response letter in May 2022, which cited issues related to chemistry, manufacturing, and controls.
Athena BDA priority score of 2 given peak potential sales of $750M. No confirmed contacts working on this asset but a large set of contacts to approach.
Athena BDA Hot Tip Alert:
We’re very hot on Axsome given these two late stage pipeline assets and Auvelity, an MDD asset which was approved in 2022.
Linerixibat – GSK – Primary Biliary Cholangitis
GSK’s investigational ileal bile acid transporter inhibitor, linerixibat, met its primary endpoint in the Phase 3 GLISTEN trial by significantly reducing itch severity in patients with cholestatic pruritus associated with Primary Biliary Cholangitis. This positive outcome positions linerixibat as a potential first-in-class therapy for this debilitating symptom.
Athena BDA priority score of 2 given unknown market potential. Multiple launch contacts identified and a wider group of hepatology leads to approach.
Pimicotinib – Merck KGaA – Tenosynovial Giant Cell Tumor
Merck KGaA’s investigational CSF-1R inhibitor, pimicotinib, achieved a 54% objective response rate at week 25 in the Phase 3 MANEUVER trial for tenosynovial giant cell tumor, compared to 3.2% with placebo. The company plans to present detailed results at an upcoming medical conference and is evaluating next steps for regulatory submissions.
Athena BDA priority score of 2 given unknown market potential. Launch lead identified with larger number of oncology contacts to also approach.
GSK’227 – GSK – Lung Cancer
The FDA has granted Breakthrough Therapy Designation to GSK’s investigational B7-H3-targeted antibody-drug conjugate, GSK’227, for treating relapsed or refractory extensive-stage small-cell lung cancer. This designation aims to expedite the development and review of drugs showing substantial improvement over existing therapies for serious conditions.
Athena BDA priority score of 2 given early status of the asset but there has been a commercialization lead assigned.
FDA Approvals Updates:
Attruby – BridgeBio – ATTR
The FDA has approved BridgeBio’s Attruby for treating transthyretin amyloid cardiomyopathy (ATTR-CM), a rare and fatal heart condition. Priced at approximately $244,000 annually, Attruby will compete with Pfizer’s Vyndaqel, which generated $3 billion in sales last year.
Athena BDA priority score of 1 given expected sales of close to $3B, large number of target contacts, and open marketing/ medical job postings.
Ziihera – Jazz – Biliary Tract Cancer
The FDA has granted accelerated approval to Jazz Pharmas’ Ziihera, a bispecific HER2-directed antibody, for adults with previously treated, unresectable or metastatic HER2-positive biliary tract cancer. In trials, Ziihera achieved a 52% objective response rate, with a median duration of response of 14.9 months.
Athena BDA priority score of 1 given potential peak sales of $2B, multiple launch leads identified and a wider oncology team at Jazz to approach.
Bimzelx – UCB – Hidradenitis Suppurativa
The FDA has approved UCB’s Bimzelx for treating moderate-to-severe Hidradenitis Suppurativa in adults, making it the first IL-17A and IL-17F inhibitor approved for this condition. This marks the fifth U.S. indication for Bimzelx, underscoring UCB’s commitment to addressing unmet needs in immune-mediated inflammatory diseases.
Athena BDA priority score of 1 given multi billion dollar peak expected sales, and many contacts identified as working on the HS launch.
Athena BDA Hot Tip Alert:
This is the 4th new indication approval for Bimzelx in the last 3 month and we’re seeing an influx of job postings in the immunology division.
Aucatzyl – Autolus – Acute Lymphoblastic Leukemia
The FDA has approved Autolus’ Aucatzyl, a CD19-directed CAR T-cell therapy, for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. This approval positions Aucatzyl to compete with existing treatments like Gilead’s Tecartus in the CAR-T therapy market.
Athena BDA priority score of 2 given peak expected sales of $300M and moderate number of launch contacts to approach.
Kebilidi – PTC Therapeutics – AADC
The FDA has granted accelerated approval to PTC’s Kebilidi, the first gene therapy in the U.S. administered directly to the brain for treating AADC deficiency, a rare and life-threatening genetic disorder. Analysts project that Kebilidi could achieve peak revenues of approximately $273 million by 2026.
Athena BDA priority score of 2 given revenue projections. Only two confirmed gene therapy leads, but plenty of contacts to target in a hotly tipped company commercializing multiple assets.
Revuforj – Syndax – Acute Leukemia
The FDA has approved Syndax’s Revuforj, the first menin inhibitor, for treating relapsed or refractory acute leukemia with KMT2A translocation in patients aged one year and older. In clinical trials, Revuforj achieved a complete remission rate of 21%, with a median duration of 6.4 months.
Athena BDA priority score of 2 given $200M peak expected sales revenue and moderate amount of launch contacts to approach, who are likely also aligned to their other 2024 launch brand, Niktimvo.
The best of the rest below in table format.